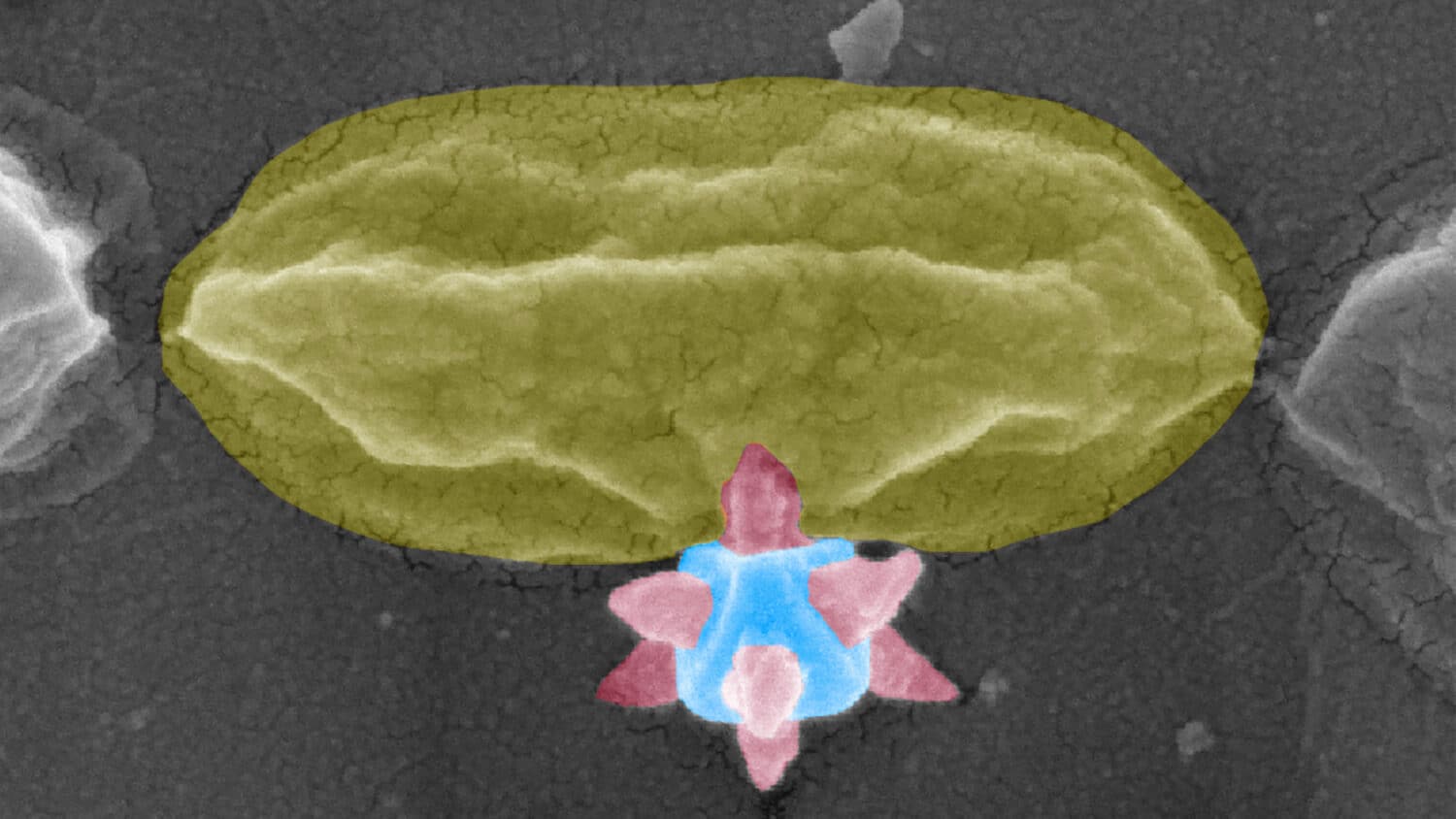
Bacteria that multiply on surfaces are a major headache in healthcare when they gain a foothold on, for example, implants or in catheters. Researchers at Chalmers University of Technology have found a new weapon to fight these hotbeds of bacterial growth – one that does not rely on antibiotics or toxic metals. The key lies in a completely new application of this year’s Nobel Prize-winning material: metal-organic frameworks. These materials can physically impale, puncture and kill bacteria before they have time to attach to the surface.
Because once bacteria attach to a surface, they start to multiply while encasing themselves in what is known as a biofilm – a viscous, slimy coating that protects the bacteria and makes them difficult to kill. Biofilms thrive particularly well in humid environments and can pose serious challenges in healthcare. For example, bacteria can attach to medical devices such as catheters, hip replacements and dental implants, and lead to hospital-acquired infections (HAI), also known as nosocomial infections – a widespread problem worldwide that causes great suffering and high healthcare costs, and an increased risk of the development of antibiotic resistance.
Biofilms can also form on ship hulls, where they can lead to troublesome algal biofouling and barnacle growth, slowing down the ship while increasing its fuel consumption. Furthermore, antifouling paints containing toxic biocides are often used on ship hulls to combat this problem, with an associated risk of harmful substances leaching into the marine environment. Biofilms in industrial piping systems are also a widespread problem that can cause corrosion, clog the systems, reduce their efficiency, and increase energy consumption for example.